Human enterohepatic adaptation of iron transport and metabolism to hypoxia
(Projektleitung O. Götze & J. Schmitt; Kooperation ZIHP)
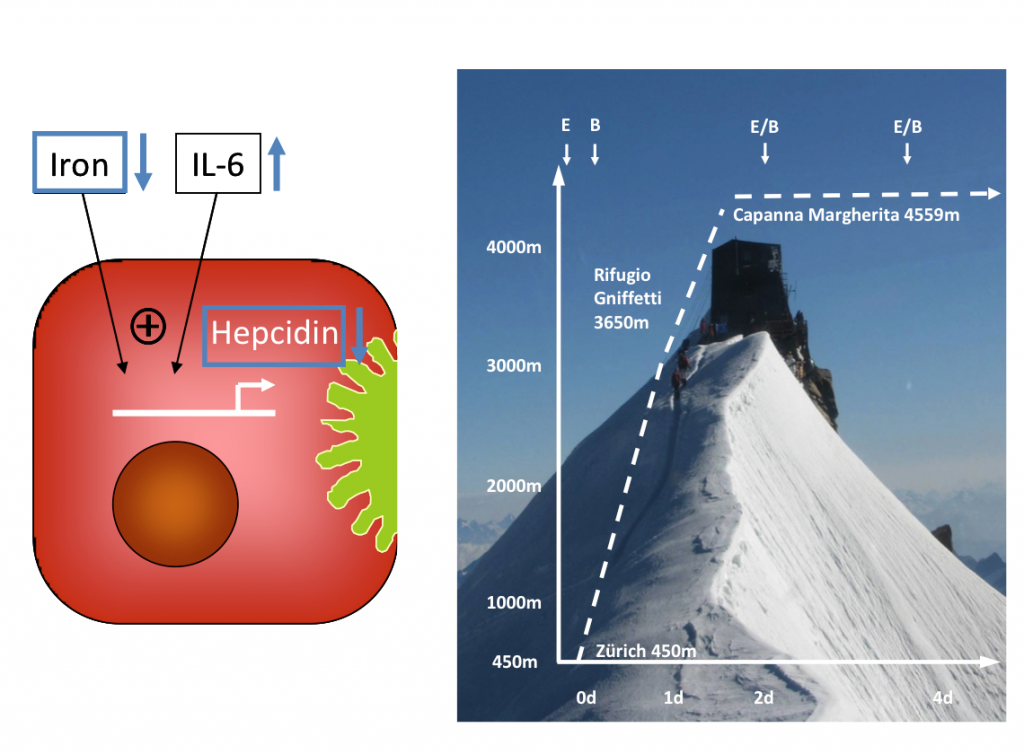
Human iron homeostasis is regulated by intestinal iron transport, hepatic hepcidin release and by signals from pathways consuming and supplying iron. The body iron content is tightly regulated, because accumulation of intracellular iron causes cell and tissue damage, presumably by iron-catalyzed generation of reactive oxygen species. Stimulated erythropoesis by hypoxia increases the iron demand in the erythropoietic compartment. Increased iron absorption during the first days of exposure to high altitudes has been identified as a central regulatory process supporting adaptive erythropoiesis five decades ago in men. To extend the mechanistic understanding of high altitude iron homeostasis beyond the level of hepcidin modulation, we aimed to characterize the adaptive enterohepatic regulation of the intestinal iron absorption process in man under hypoxemic conditions which has not been characterized in a comprehensive approach to date. This study represents the analysis of adaptive enterohepatic regulation of intestinal iron absorption under hypoxic conditions and is part of a cooperative project (principal investigators: M. Maggiorini & Th. Lutz) supported by the Zurich Centre for Integrative Human Physiology (ZIHP). The aim of this study was to characterize the adaptation of iron homeostasis under acute hypoxia in man at the levels of (i) intestinal iron transport (ii) hepatic hepcidin release (iii) and of systemic inflammatory and erthropoetic responses. The study uncovers the intestinal regulatory mechanisms underlying adaptive changes in iron metabolism under hypoxic conditions for the first time in humans.
Enterohepatic Fgf15/19 signaling during intestinal inflammation and consecutive changes in bile acid metabolism
(Projektleitung M. Rau; Kooperation G. Rogler, Zürich)
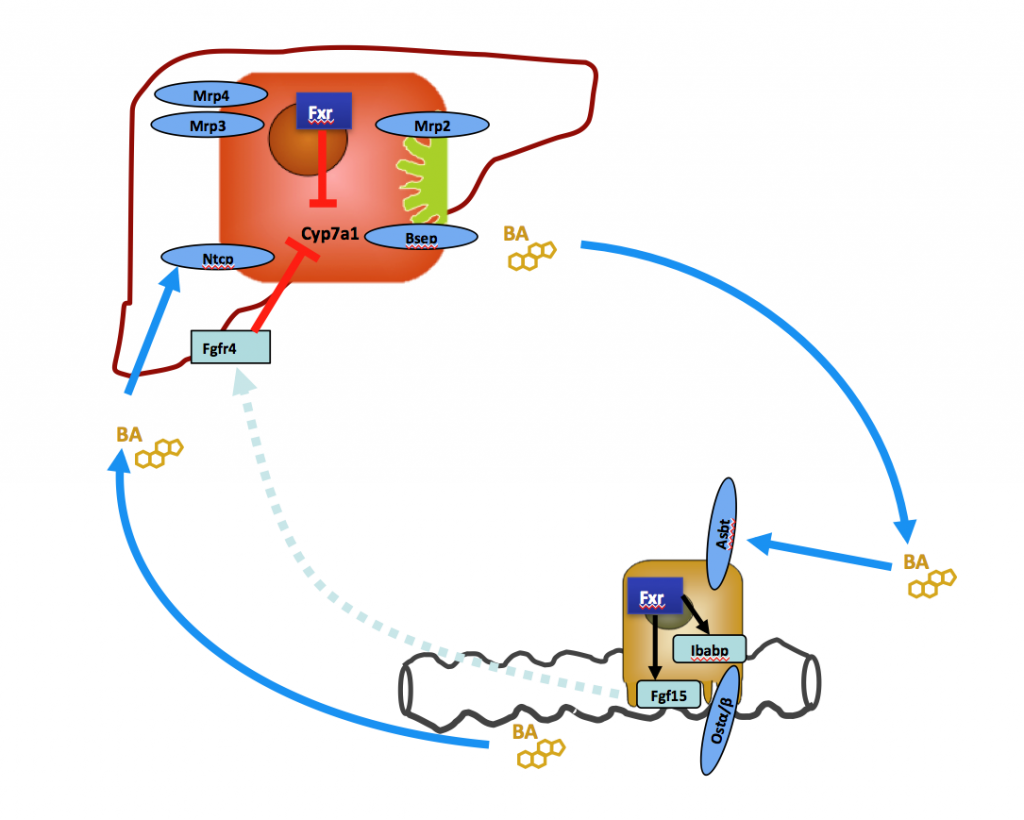
Inflammatory bowel disease is frequently associated with chronic cholestatic liver disease affecting intra- and extrahepatic bile ducts. The pathogenetic pathway leading from intestinal inflammation to cholestatic liver disease is still poorly understood. We currently study alterations in enterohepatic Fgf15/19 signaling in different forms of intestinal inflammation and associated changes in hepatic bile acid metabolism and bile composition.
Role of bile acids and vitamin D in the pathogenesis of alcoholic liver disease
(gefördert durch die Hartmann-Müller-Stiftung Zürich)
(Projektleitung J. Schmitt)
Increased serum bile salt levels have been associated to a single nucleotide polymorphism in the bile salt export pump in several acquired cholestatic liver diseases but not yet in alcoholic liver disease (ALD). Furthermore, a cross-talk between vitamin D and bile acid synthesis has recently been discovered. Aim of this project is to analyze the role of genetic polymorphisms in the bile salt export pump (BSEP; ABCB11) and the vitamin D receptor gene (VDR; NR1I1) on the emergence of cirrhosis in patients with chronic alcoholic liver disease.