Effects of bile acid retention on the natural course of HCV infection
(Projektleitung M. Rau)
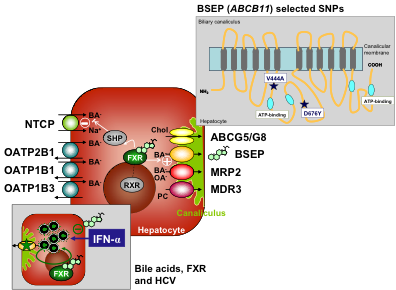
Overview of key transporters involved in hepatocellular bile formation.
Bile salts are predominantly taken up by NTCP and to a minor extent by the OATPs, which work sodium-independent. OATPs are also key drug uptake systems. Bile salts are secreted into the canaliculus by the BSEP, while the biliary lipids phosphatidylcholine and cholesterol require MDR3 and the heterodimeric transporter ABCG5/ABCG8, respectively. In normal physiologic states, the nuclear receptor FXR acts as bile salt sensor and downregulates the expression of NTCP indirectly and upregulates expression of BSEP directly. Single nucleotide polymorphisms of the BSEP (ABCB11) gene with clinical implications for drug-induced cholestasis, intrahepatic cholestasis of pregnancy and viral hepatitis C are displayed in a topographical scheme (right upper insert). In livers infected with HCV, bile salts interfere with IFN signaling and promote HCV replication in an FXR-mediated manner (left lower insert).
Role of 25-OH-Vitamin D and common Vitamin D Receptor (NR 1I1) polymorphisms in the fibrogenesis and therapy response in chronic hepatitis C patients
(Projektleitung: K. Baur, A. Geier)
The vitamin D receptor (VDR) pathway mediates antiinflammatory and antifibrotic effects in hepatic stellate cells. In chronic HCV patients, 25-OH-vitamin D levels have been associated with stage of fibrosis and inflammation. We recently examined whether VDR gene polymorphisms (SNP) and low serum vitamin D lead to altered hepatic gene expression and rapid fibrosis progression in these patients. Low 25-OH vitamin D plasma levels and the unfavourable VDR bAt[CCA]-haplotype are associated with rapid fibrosis progression in chronic HCV patients. Interestingly, the same vitamin D receptor polymorphisms influence also the response to PEG-interferon/ribavirin based therapy and exerts an additive genetic predisposition to low 25-OH vitamin D serum levels.
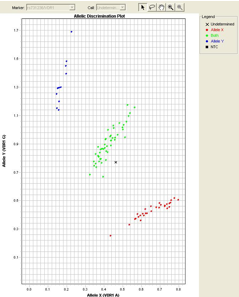 Single Nucleotide Polymorphism (SNP) Genotyping
Single Nucleotide Polymorphism (SNP) Genotyping
Impact of genetic SLC28 transporter and ITPA variants on side effects and therapeutic response in patients with HCV infection
(Projektleitung M. Rau)
Treatment of chronic hepatitis C is evolving, and direct acting antivirals (DAAs) are now added to pegylated interferon-α (Peg-INF-α) and ribavirin (RBV) for the treatment of hepatitis C virus (HCV) genotype 1 infection. DAAs cause different side effects and can even worsen RBV induced hemolytic anemia. Therefore, identifying host genetic determinants of RBV bioavailability and therapeutic efficacy will remain crucial for individualized treatment. Recent data showed associations between RBV induced hemolytic anemia and genetic polymorphisms of concentrative nucleoside transporters such as CNT3 (encoded by SLC28A3) and inosine triphosphatase (encoded by ITPA).
We therefore analyze the association of genetic variants of SLC28 transporters and ITPA with RBV induced hemolytic anemia and treatment outcome. Preliminary data on RBV induced hemolytic anemia and treatment response underscore the need for further studies on host genetic determinants of RBV bioavailability and therapeutic efficacy for individualized treatment of chronic hepatitis C.